Partnering with BioVigil to Increase Hand Hygiene Compliance

Partnering with BioVigil to Abate C. Diff and Increase Hand Hygiene Compliance
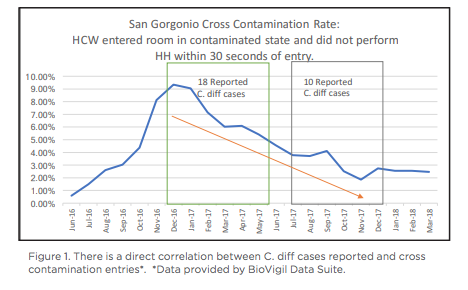
San Gorgonio Memorial Hospital is a 72-bed acute care hospital nestled in Banning California, about a forty minute drive from Palm Springs. San Gorgonio Memorial serves the small communities of San Gorgonio Pass. For the last three years, San Gorgonio Memorial received Inland Empire’s top workplaces award. As impressive as the award is, even a top-rated community hospital can still be impacted by Healthcare Acquired Infections (HAIs), including Clostridium Difficile (C. diff). Susan Sommers, Director of Infection Prevention and Risk Management, began her career at San Gorgonio in March 2017, one year after BioVigil had been installed and was not being used effectively. Sommers was motivated to understand BioVigil’s capabilities, since she had never been exposed to an electronic hand hygiene solution. She dedicated time to work with IT to learn and understand the value of Biovigil and how to use it. Her next step was to earn the support of staff and leadership, and then train and on-board those teams. Sommers shared that not every hospital has access to an electronic hand hygiene solution: “I felt lucky that we had such cool technology in our community hospital and wanted to see what would happen if we used it correctly.” After working with IT and executive leadership, Sommers was ready to relaunch BioVigil. Around the same time that San Gorgonio started effectively using BioVigil, they also made two other changes to hospital processes to reduce C. diff. They moved from polymerase chain reaction (PCR) testing, which is highly sensitive, to Enzyme-linked immune sorbent assay (ELISA) testing. PCR tests used on patients will show positive results, whether they have been infected or not. ELISA is only positive if a patient has C. diff. In addition, San Gorgonio decided to test Bristol 7 stools within the first 72 hours after a patient’s admission. Testing Bristol 7 stools before the 72 hours would be deemed as a community infection, as opposed to testing after 72 hours which would be considered a healthcare-acquired infection. With a considerable amount of analysis and testing, coupled with BioVigil, C.diff was drastically reduced in six months.

Exceeding Compliance and HAI Expectations
Sommers’ expectations of San Gorgonio’s hand hygiene compliance potential hovered around 85%, simply because she didn’t believe that achieving 85% was even possible. Instead, hand hygiene compliance increased from 65% (direct observation prior to BioVigil installation) to 92%. Figure 2 depicts San Gorgonio’s compliance prior to BioVigil at 65% (based on their data). The month BioVigil was implemented, there was a spike in compliance because of the new program. Thereafter, compliance and usage dropped until Sommers came on board. Her passion and commitment for their hand hygiene initiative steadily increased compliance and exceeded her expectations. Best Practices, Processes and Rewards Sommers integrated BioVigil training as part of new hire orientation at San Gorgonio Memorial. They also placed table tents in common areas, so visitors and patients can quickly understand what BioVigil is and the value it provides, as well as the San Gorgonio teams’ dedication to patient safety. Each department is also rated on their hand hygiene compliance. The department with the highest compliance became the highest because BioVigil was incorporated in their individual staff evaluations. Sommers has a unique approach for healthcare workers who don’t feel they need to be monitored by an electronic hand hygiene solution. She tells them, “You are already washing your hands. You might as well get credit for it.” The best performers are identified in the reports delivered by BioVigil Data Suite and recognized by each department. The San Gorgonio Medical Surgery team recently had a pizza party for exceeding 90% hand hygiene compliance. As for the large reduction in C.diff cases, Sommers expects them to continue decreasing.